Bringing Product Ideas to Life
EMERGE
The roadmap to our product life cycle
Whether we’re working on internal product lines, collaborating with business partners, or consulting with inventors, we deploy the EMERGE process to power medical device innovation—transforming ideas into market-ready products.
Quality From The Start
Featuring dozens of 510(K)s and patents, we have experience at all stages of product development, including clinical manufacturing and regulatory approval. The MedSource team is ready to provide critical support from idea conception to a successful new product launch.

Design controls
Ongoing consistency and quality monitoring are paramount to continued operations, so every EMERGE process begins with a focus on how design and quality will be evaluated throughout the product lifecycle.

Regulatory Compliance
In-house experts specialize in navigating and complying with all FDA and international certification requirements.
From the early planning stages, we apply a long-term vision for regulatory approval and compliance.
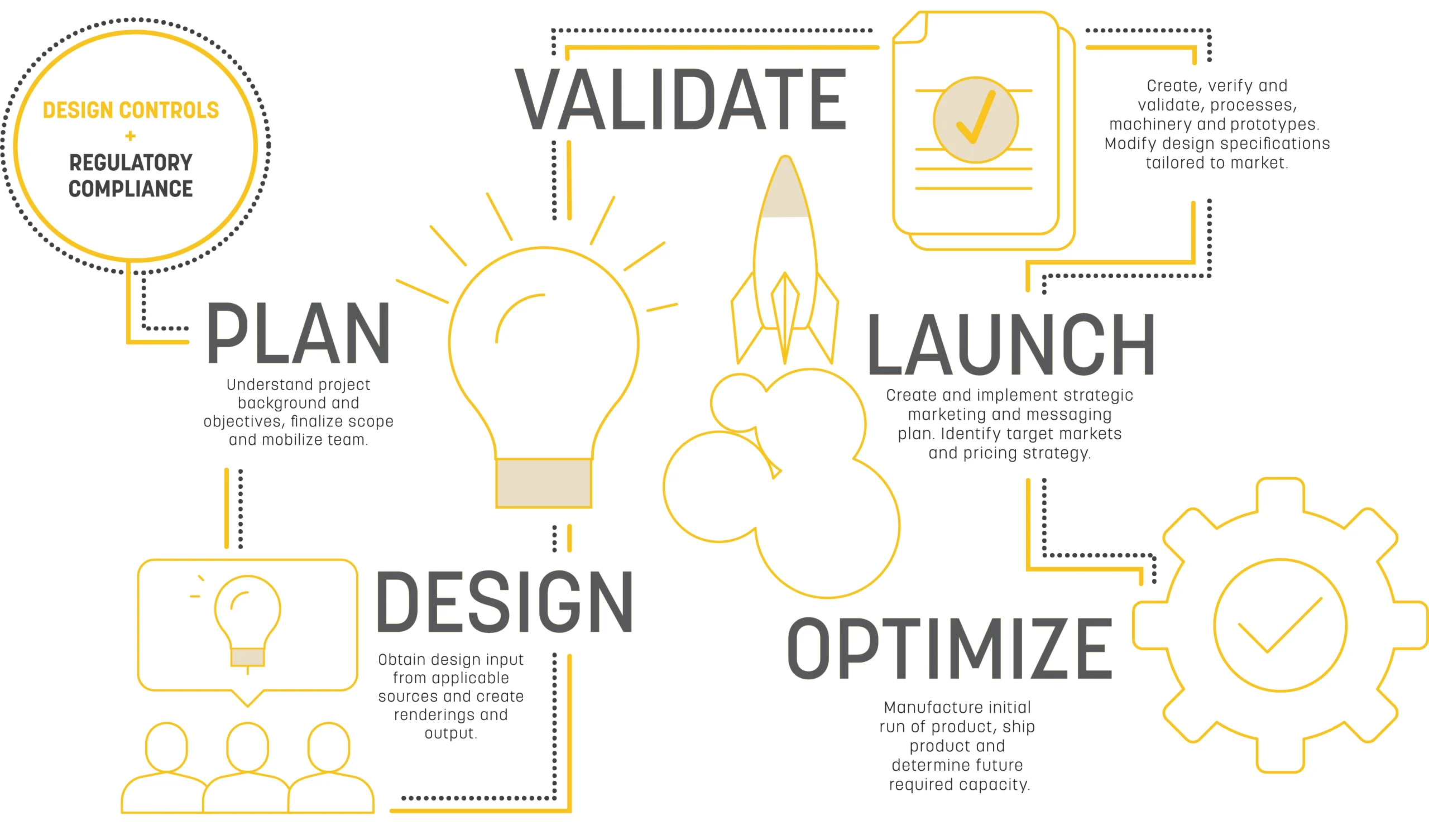
Emerge Process Steps

Plan
Understand the project background and objectives, perform market research, finalize scope, and mobilize the team.

Design
Obtain design input from multiple sources, such as vendors, end users, and industry experts. Create renderings and begin to define the output requirements.

Validate
Create, verify, and validate manufacturing processes, including machinery and first run prototypes. Modify design specs as needed to refine the model or tailor to market needs.

Optimize + Scale
Manufacture the initial run of the product, ship, and determine future capacity requirements.
Private Brands
& OEM Partnerships
MedSource Labs offers private label opportunities and product customization tailored to individual business needs.
We also welcome OEM partnerships—collaborating to develop and manufacture high-quality medical products that align with your brand and goals. Let’s work together to bring innovative solutions to market.
